Johlin JM. 1930. The influence of pH and solution concentration on the surface tension of gelatin solutions determined by the sessile bubble method. J Biol Chem 87:319-325.
obtained by the sessile bubble method of measuring surface tensions
Johlin JM. 1930. The influence of pH and solution concentration on the surface tension of gelatin solutions determined by the sessile bubble method. J Biol Chem 87:319-325.
obtained by the sessile bubble method of measuring surface tensions
A Thermodynamic Model for Low Interfacial Tensions in Alkaline Flooding. Sharma, Mukul M.; Yen, T.F. SPE Journal. Volume 23, Number 1. February 1983
Many experimental studies have been undertaken to measure interfacial tensions (IFT’s) as a function of pH, salinity, temperature, and divalent ion concentrations.
The molecular approach involves a statistical mechanical calculation of the intermolecular forces operating at the interfaces between two phases.
This citing is probably too old (1983)
Effect of pH on the Interfacial Tension of Lipid Bilayer Membrane. Aneta D. Petelska and Zbigniew A. Figaszewski. Biophysical Journal, Volume 78, Issue 2, 812-817, 1 February 2000.
A theoretical equation is derived to describe the dependence of the interfacial tension of a lipid bilayer on the pH of the aqueous solution. Interfacial tension measurements of an egg phosphatidylcholine bilayer were carried out. The experimental results agreed with those derived from the theoretical equation obtained close to the isoelectric point within a range of three pH units. A maximum corresponding to the isoelectric point appears both in the theoretical equation and in the experiment.
Sensitivity analysis of interfacial tension predictions for hydrocarbon fluids. DANDEKAR Abhijit Y. Petroleum science and technology. 2004, vol. 22, no9-10, pp. 1161-1172 [12 page(s) (article)] (11 ref.)
The interfacial tension (IFT) of hydrocarbon fluids is commonly predicted by either the parachor method or the scaling law. The methods require equilibrium liquid and vapor phase composition and density. An equation of state would normally be required if experimental values are not available. However, the computation of density for simple hydrocarbons and reservoir fluids, despite the important advances achieved by cubic equations of state, still remains a weak link in these types of calculations. Thus, there exists a need to investigate the qualitative and quantitative effects, of such inaccuracies in the density, on IFT predictions. Moreover, the study presented in this work would be useful in reservoir engineering and enhanced oil recovery calculations. The results presented in this work indicate that the methods are highly sensitive to the inaccuracies in the density of both the liquid and the vapor phases. An error of around 10% in the liquid or the vapor density can result in an error of up to 200% in the estimated IFT. Two binary and one ternary mixture for which measured data on IFT, composition and density is reported in the literature form the basis of this study.
Neural Network Prediction of Interfacial Tension at Crystal/Solution Interface. K. Vasanth Kumar. Ind. Eng. Chem. Res., 2009, 48 (8), pp 4160–4164
Using (1) solubility, (2) molecular weight, and (3) density, a three-layer feed-forward neural network was constructed and tested to predict the IFT at the crystal/solution interface. The concentration of solute in liquid phase, (1) concentration of solute in solid phase, (4) temperature, (3) density and (2) molecular weight of crystal were used as inputs to predict the interfacial tension at the crystal/liquid interface (σSL). The network was trained using the solubility information for 28 systems to predict the σSL value and was validated with 29 new systems. Despite the limited number of data used for training, the neural network was capable of predicting σSL successfully for the new inputs, which are kept unaware during the training process. The σSL value that is predicted by the artificial neural network during the training and testing process was compared with σSL predicted from the widely used empirical expression. For most of the systems, ANN better predicts IFT.
Bahasa Indonesia for output is keluaran. Many people mistakenly use completely different meaning luaran instead. Check KBBI
pH is a measure of the acidity or basicity of a solution, defined as minus the decimal logarithm of the hydrogen ion activity in a solution.
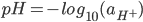

common pH scale (can't find where water is?)
Kamus Besar Bahasa Indonesia (KBBI) (main dictionary reference for Indonesian Language)
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear stress or tensional stress. In everyday terms (and for fluids only), viscosity is “thickness”. Thus, water is “thin”, having a lower viscosity, while honey is “thick”, having a higher viscosity.
Viscosity describes a fluid’s internal resistance to flow and may be thought of as a measure of fluid friction. See Newton’ expression: the constant μ is known as the coefficient of viscosity, the viscosity, the dynamic viscosity, or the Newtonian viscosity.
What are cross-validation and bootstrapping?
Cross-validation and bootstrapping are both methods for estimating generalization error based on “resampling”.
In k-fold cross-validation, you divide the data into k subsets of (approximately) equal size. You train the net k times, each time leaving out one of the subsets from training, but using only the omitted subset to compute whatever error criterion interests you. If k equals the sample size, this is called “leave-one-out” cross-validation.