Young Laplace equation:
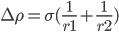
other uses γ instead of σ for surface/interfacial tension (i.e. Pashley 2004). Common unit is dyne/cm (1 dyne = 1 micro Newton (μN) ).
Young Laplace equation:
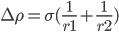
other uses γ instead of σ for surface/interfacial tension (i.e. Pashley 2004). Common unit is dyne/cm (1 dyne = 1 micro Newton (μN) ).
organic chemistry of surfactant:
CH3(CH2)nCH2S
"tail" "head"
hydrophobic hydrophilic
(Section 1.7) Presently, about 50% of the surfactants used in the surfactant industry are derived from petrochemical raw materials, and the other 50% are derived from oleochemical raw materials.
Renewable surfactant feedstocks are often perceived as being better for the environment and should therefore be the first choice for environmentally ‘‘friendly’’ products. But is that ‘‘analysis’’ of the situation scientific fact or spiritually pleasing fiction?
From biodegradation, removal by sewage treatment, toxicity, and similar studies indicate that there is little or no measurable difference between surfactants based on petrochemical and renewable raw materials in terms of their direct impact on the environment.
Surfactants may also cause problems at later stages of oil processing. In some cases, especially where the extracted crude is recovered in the presence of a great deal of water, the presence of surfactants produces emulsions or microemulsions that must be broken and the water separated before further processing can occur. Naturally present surface-active materials in the crude plus any added surfactants can produce surprisingly stable emulsion systems. The petroleum engineer dilemma: (1) surfactants are necessary for efficient extraction, (2) but their presence produces difficult problems in subsequent steps.
Processes such as steam flooding involve injecting high-pressure steam at about 340° C into the oil bearing rock formations. The steam heats the crude oil, reducing its viscosity and applying pressure to force the material through the rock matrix toward recovery wells. Unfortunately, the same changes in the physical characteristics of the crude oil that make it more mobile in the formation also render it more susceptible to capillary phenomena that can cause the oil mass to break up within the pores of the rocks and leave inaccessible pockets of oil droplets. In such processes, surfactants are used to alter the wetting characteristics of the oil–rock–steam interfaces to improve the chances of successful recovery. Those surfactants must be stable under the conditions of use such as high temperatures and pressures and extremes of pH.
Myers D. 2006. Surfactant Science and Technology. Ed ke-3. New Jersey: J Wiley.
(1) Proper wetting of oil-bearing formations, (2) microemulsion formation and solubilization properties, (3) ease of emulsion breaking after oil recovery.
The properties and applications of surfactants are determined by the balance between the lyophilic (‘‘solvent-loving’’ and lyophobic (‘‘solvent-hating’’) portions of the molecules. (There are also water-loving hydrophilic, water-hating hydrophobic, fat-loving lipophilic, and fat-hating lipophobic).
For that reason, such characteristics as solubility, surface tension reducing capability, critical micelle concentration (cmc), detergency power, wetting control, and foaming capacity may make a given surfactant perform well in some applications and less well in others. The ‘‘univer-
sal’’ surfactant that meets all the varied needs of surfactant applications has yet to emerge from the industrial or academic laboratory.
Surfactant Science and Technology
3rd edition. Myers, Drew 2006
John Wiley & Sons, Inc. Hoboken, New Jersey, USA.Surfactant_Science_and_Technol.pdf