A spectrophotometer is a photometer (a device for measuring light intensity) that can measure intensity as a function of the color (or more specifically the wavelength) of light.
Arif
Arif
Surface Tension Prediction for Pure Fluids
Joel Escobedo and G. Ali Mansoori. AIChE Journal, Volume 42, No. 5, pp. 1425-1433, May 1996. (this work is cited in their next work: Escobedo 1998)
Arif
Escobedo and Mansoori (AIChE J. 42(5): 1425, 1996) Found original paper: check Escobedo 1996
The proposed 1996 paper relates the surface tension of mixtures to bulk-phase concentrations and properties (i.e. densities). Surface tension of pure organic fluids σ:
![\sigma=[P(\rho_l-\rho_v)^4]](http://lakm.us/thesit/wp-content/uploads/eq_90c5333f1eb13eb375ab39bad2a55fd3.png)
The two ρ are densities: liquid and vapour.
They introduced parachor (P), a new expression, derived from statistical mechanics (from Macleod equation) which represents the experimental surface tension of 94 different organic compounds within 1.05 AAD% (average absolute deviations).
(Kumar 2005) It expresses the surface tension of a liquid in equilibrium with its own vapour. Historical reference for parachor cited here: Macleod, Sugden, Quayle, Escobedo.
Arif
Surface Tension Prediction for Liquid Mixtures
Joel Escobedo and G. Ali Mansoori.AIChE Journal, Vol. 44, No. 10, pp. 2324-2332, 1998.
Surface tension of pure organic fluids was proposed previously also by them in 1996. The same theory is extended to the case of mixtures of organic liquids.
Arif
Young Laplace equation:
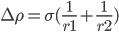
other uses γ instead of σ for surface/interfacial tension (i.e. Pashley 2004). Common unit is dyne/cm (1 dyne = 1 micro Newton (μN) ).
Arif
organic chemistry of methyl ester sulfonate (MES) surfactant:
RCH(CO2Me)SO3Na
"tail" "head"
hydrophobic hydrophilic
Anionic: the hydrophile is a negatively charged group i.e. sulfonate RSO3- M+
Arif
organic chemistry of surfactant:
CH3(CH2)nCH2S
"tail" "head"
hydrophobic hydrophilic
Arif
(Section 1.7) Presently, about 50% of the surfactants used in the surfactant industry are derived from petrochemical raw materials, and the other 50% are derived from oleochemical raw materials.
Renewable surfactant feedstocks are often perceived as being better for the environment and should therefore be the first choice for environmentally ‘‘friendly’’ products. But is that ‘‘analysis’’ of the situation scientific fact or spiritually pleasing fiction?
From biodegradation, removal by sewage treatment, toxicity, and similar studies indicate that there is little or no measurable difference between surfactants based on petrochemical and renewable raw materials in terms of their direct impact on the environment.
Arif
Surfactants may also cause problems at later stages of oil processing. In some cases, especially where the extracted crude is recovered in the presence of a great deal of water, the presence of surfactants produces emulsions or microemulsions that must be broken and the water separated before further processing can occur. Naturally present surface-active materials in the crude plus any added surfactants can produce surprisingly stable emulsion systems. The petroleum engineer dilemma: (1) surfactants are necessary for efficient extraction, (2) but their presence produces difficult problems in subsequent steps.
Arif
Pashley RM, Karaman ME. 2004. Applied Colloid and Surface Chemistry. West Sussex: J Wiley.